Seven Ways Compass MED Helps Speed Time to Market for Med Device Manufacturers
Medical Device | Risk Management | Compass | Design | compliance | Regulations
Medical device manufacturers face significant pressures to bring safer products to market faster. However, meeting critical project milestones and launch dates on time and within budget is becoming increasingly difficult. Medical devices are becoming more complex—which makes product development more difficult. At the same time, industry standards and regulations are evolving, placing new pressures on quality, regulatory, and engineering teams.
As we’ve watched these challenges unfold and intensify, we’ve made it a top priority to equip manufacturers with a solution that can help address these challenges:
|
“We don’t believe the path to a med device product launch is going to get any easier, any time soon, but we do believe that significant, untapped opportunities can help accelerate the journey.” |
- We’ve spent hundreds of hours exploring common sources of development inefficiencies, focusing on requirements, risk, and test management;
- We’ve interviewed and piloted new approaches with dozens of executives, engineers, and quality leaders, and;
- We’ve met with customers one-on-one, identifying new features that could enhance efficiencies, collaboration, and quality.
What is the result of all of that exploration and research? Compass MED.
This solution was created and designed specifically for medical device manufacturers. It contains all of the critical capabilities that made our original Compass solution so beneficial to medical device manufacturers (including real-time traceability, guided design controls, submission-ready documents, and change once-update everywhere functionality), but it brings even more capabilities when it comes to supporting enhanced efficiency, collaboration, and quality.
Here are seven of the Compass MED features I’m most excited about for medical device manufacturers:
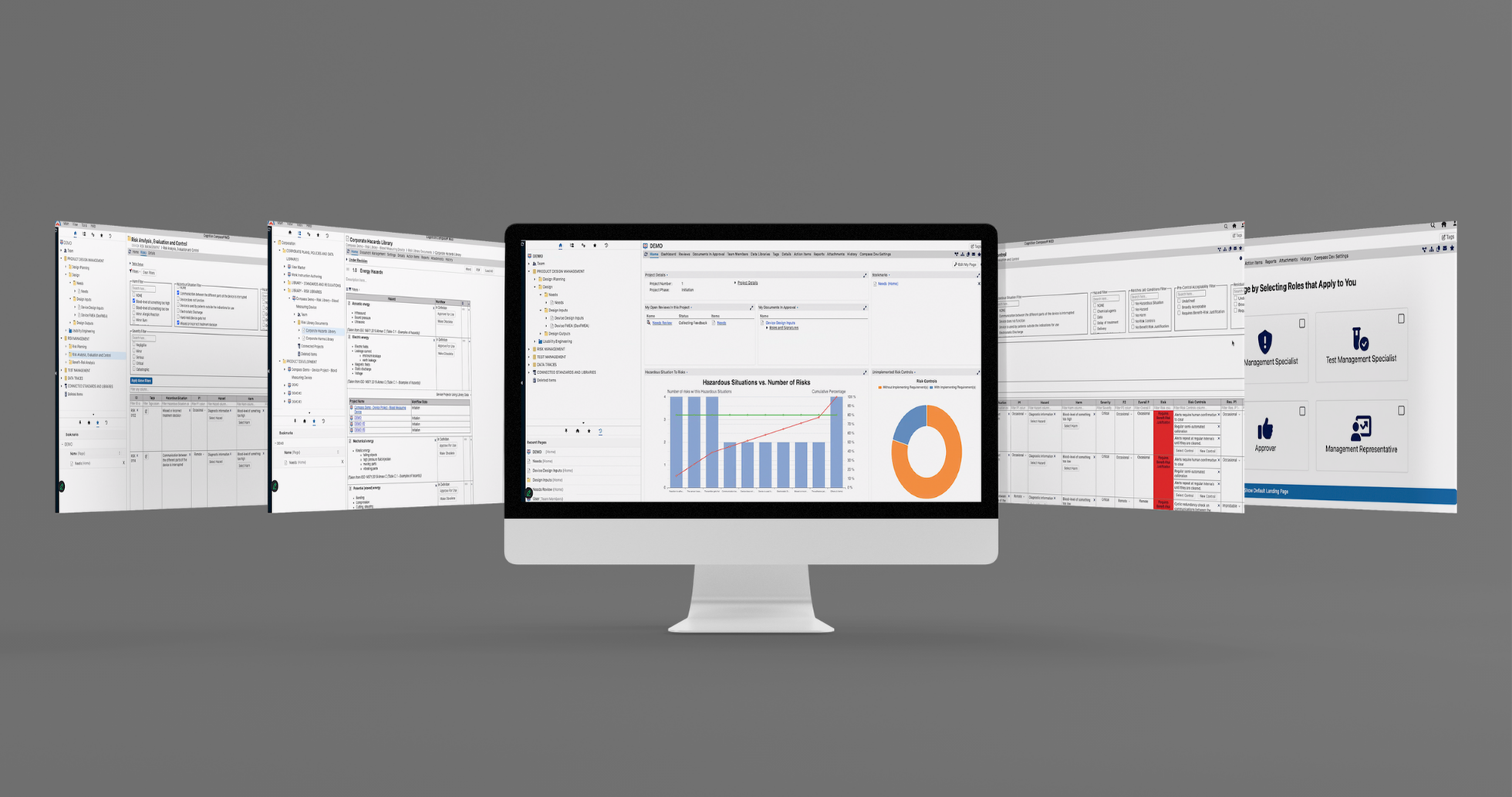
1. Flexible workspaces for both design controls and risk management
When individuals can view and organize data in the ways that make the most sense for them, they can work much more efficiently. This is why we added flexible workspace capabilities in Compass MED.There are several applications for flexible workspaces, including the ability to:
- Create one view with multiple data connections
- Create customized tables
- Reorder columns, hide or show columns, and change column width
- Filter information, such as by keyword, tag, document, or related data
- Review specific requirements and risk data items
- Access real-time filters to identify critical details (such as mitigations without controls)
To ensure data integrity and quality, information entered into workspaces instantly becomes part of the document. This enables users to create workspaces without manipulating export data format, and it eliminates the risk of cut-and-paste errors. In other words, data can be presented in different formats without affecting the exportable record.
2. User-defined landing pages
To further enhance efficiencies, we’ve made it easy for Compass MED users to create their own landing pages based on their unique preferences. As a result, users can instantly access what they need most, providing significant time-savings.
For example:
- A project lead might set up a landing page that shows the project status in a single view that enables them to jump to the item to review, manage, and approve as needed.
- An approver might set up a landing page to instantly see what documents are ready for them to review.
- An author might set up a landing page to instantly see and access relevant documents and workspaces.
- A tester might set up a landing page to review which tests are in their queue.
- A risk specialist might set up a page to quickly review open risk items and documents and add reports.
3. Interactive dashboards
Flexible workspaces and user-defined landing pages help users quickly identify what’s most relevant to them, yet Compass MED further enhances efficiency by supporting reporting dashboards.
We often describe dashboards as a way to visualize the status of key deliverables in a consolidated and easy-to-digest way. Users can explore critical project information using the dashboards and see a snapshot of the project status at a glance, with a focus on key areas.
There are several use cases for dashboards, but some of the most beneficial applications may include:
- Summarizing project status and open activities via graphical representation of the workflow state
- Determining if all requirements have been tested
- Identifying if upper-level requirements (needs or design inputs) lack lower-level requirements (and ensuring all outputs and inputs are satisfied)
- Determining if all risk controls have been defined and implemented into the product design
- Identifying if risk controls are controlling multiple risks
- Determining which hazards are the most significant contributors to overall risk
4. Integrated comprehensive risk management
Due to the evolving regulatory requirements and high stakes associated with quality and data integrity, one of our top objectives for Compass MED was to support and advance manufacturers’ risk management capabilities. To that end, we’ve built on our robust risk management capabilities that integrate multiple pathways (usability analysis, FMEA, use error analysis, preliminary hazard analysis, product risk analysis, and normal use or side-effect analysis) to determine hazardous situations, hazards, and harms. As a result, Compass MED supports complex risk assessment and efficient data management in a central area for comprehensive analysis.
Here are some specific ways Compass MED’s integrated risk-management capabilities can benefit users:
|
“With a comprehensive risk assessment users will have accumulated hundreds of data row entries, and they will need to determine which risks are not mitigated. With Compass MED, they can easily view this data in a workspace or interactive dashboard.” |
- Determine what risks are possible via correct use, foreseeable misuse, failures due to design or use errors
- Identify which hazardous situations are recurring and assess if they can be mitigated
- Filter results to find information by keyword or by pre-built filters (such as for missing mitigations)
With Compass MED, risk is managed organically throughout the project. It starts with the preliminary hazard analysis, continues through use definition and requirements to determine points of failure, and culminates in a comprehensive product risk analysis.
5. Collaborative document reviews
Compass MED makes it easier for multiple users to collaborate within the same files, without jeopardizing data integrity.
Here are some ways Compass MED’s collaborative review capabilities can benefit users:
- Users can easily access and review the latest version of documents at any time (and rest assured that the data within them is accurate and up-to-date).
- Since reviews can easily be accomplished within the software, users no longer need to export and pass around documents for further review and edits. This eliminates the risk of errors and saves time.
- Since all documents are stored and easily accessible for reviews within the software, users no longer need to spend time hunting down the latest version of exported documents.
- Since all documents associated with a project remain within the software and are continually updated within it, users no longer need to spend time collating documents at the conclusion of projects.
Compass MED also automatically creates an audit record for any change or revision made to a document, further enhancing efficiency and collaboration and reducing the risk of errors.
6. Better integration of libraries across projects
Identifying opportunities to reduce repetitive work—or build on previous work—can lead to significant time savings. This is one reason we focused on enhancing library integrations within Compass MED.
The solution enables users to easily pull information from libraries into development documents, ensuring consistency, enhancing efficiencies, and eliminating repetitive work.
For example:
- When multiple projects have similar indications for use and the same associated harms and hazards, users can apply the same harms and hazards across projects.
- When a product must meet specific criteria from a standard or regulation, users can apply the same clauses across projects. New revisions of a standard can be incorporated into the project when needed.
- When a severity of harm is set at the organizational level, users can apply that severity across projects. This ensures consistency and prevents project-to-project discrepancies.
7. Submission-ready documents
Most medical device development platforms use a tabular data-centric approach. Users can access tables and forms to view the data, and data is entered without concern for document format or export. As a result, these platforms often fall short when it comes to exporting the documents required for an audit or regulatory submission.
In contrast, Compass MED supports both document-centric and tabular data-centric approaches. Therefore, documents can be fully authored and edited within the platform. As soon as documents are exported no post-editing required.
This provides multiple benefits, including:
- Time and resource savings, as users can confidently export documents from the system with no additional editing needed post-export
- Enhanced data integrity and alignment, as the data within the document is 100% consistent with the data in the system (since no editing needs to take place post-export)
Accelerating Your Device Development Journey
Ease of use can be a significant source of time savings and enhanced efficiencies, and we took that seriously when developing Compass MED. In addition to the above features and functions, we created a user-friendly interface that further enhances productivity and improves the user experience. We welcome the opportunity to show you the interface and explore how Compass MED can help your organization navigate the increasing market pressures and evolving regulatory requirements.
See How Compass MED Can Work for You!
Request a Demo Today.
About Ben Higgitt
Ben Higgitt is the Engineering Technical Lead at Cognition Corporation, a SaaS company that develops product development and compliance solutions for the life sciences industry. He is passionate about aligning technology with constantly evolving standards and regulatory requirements and works closely with customers, industry leaders, and regulatory experts to challenge historical precedent and design solutions that go beyond today’s requirements.