Accelerate Medical Device Development with Systems Engineering
Systems engineering should be used more often in medical device product development. This may be a strong statement, but it can be transformative for device development through decreased development costs, streamlining of interactions with the FDA, and reduced time-to-market.
We would like to address this through a series of articles on systems engineering role in Medical Device Product Development of which this is the first.
For many, the phrase “systems engineering” is typically associated with large aerospace and defense companies; companies making products that are developed over many years, with long lives in the market, extremely high costs, and a relatively low total number of products manufactured. Many of these companies also employ “large,” permanent teams of systems engineers, which could be daunting to a small device company. Few device engineers are seen at conferences on systems engineering. Instead, these events include transportation, infrastructure, government, space, and of course aerospace and defense. A casual observer might think that systems engineering is not intended for the medical device industry. This is unfortunate!
In a series of articles, we will address various parts of systems engineering from higher-level thinking about the overall approach down to detailed looks at specific systems engineering activities and actions. Our goal is to bring more awareness of systems engineering to the medical device development community and hopefully encourage companies to begin implementing systems engineering and systems thinking into their product development process.
Systems Engineering: Definition
Before defining systems engineering , we must first define a system. ISO/IEC/IEEE 15288:2015(E) defines a system as “…man-made, created and utilized to provide products or services in defined environments for the benefit of user and other stakeholders” (5.2.1 Systems). The standard states that systems may include elements from hardware, software, data, humans, processes, procedures, facilities, materials, and naturally occurring entities. To the user, a system is often considered a product or service.
The International Council on Systems Engineering (INCOSE) refers to a system as “… an integrated set of elements, subsystems, or assemblies that accomplish a defined objective. These elements include products (hardware, software, firmware), processes, people, information, techniques, facilities, services, and other support elements.”
A system is comprised of a set of system elements. Elements are discrete parts of a system. The embedded software controlling user interaction in a medical device can be considered an element of the overall system. A test strip may be considered an element of the home diabetes monitoring system.
With an understanding for the word system, we look at the phrase “systems engineering.” INCOSE defines systems engineering as “…an interdisciplinary approach and means to enable the realization of successful systems. It focuses on defining customer needs and required functionality early in the development cycle, documenting requirements, and then proceeding with design synthesis and system validation while considering the complete problem: operations, cost and schedule, performance, training and support, test, manufacturing, and disposal. Systems engineering integrates all the disciplines and specialty groups into a team effort forming a structured development process that proceeds from concept to production to operation. Systems engineering considers both the business and the technical needs of all customers with the goal of providing a quality product that meets the user needs” (INCOSE Systems Engineering Handbook, 2004).
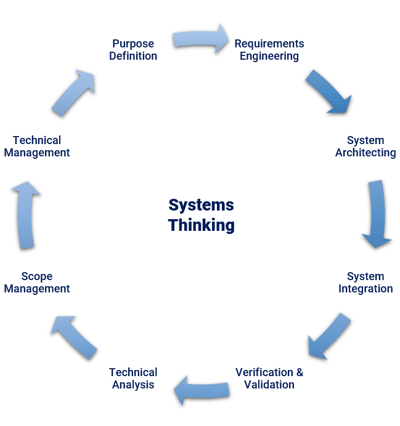
Systems engineering entails systems thinking. Systems thinking is the approach where the overall system (product), comprised of system elements, is developed and understood with a view towards how the system will be used and managed in daily life by users in various use cases and environments.
A systems engineering approach to device development, using systems thinking, helps manage the complexity of products, the regulatory environment in which they are developed and used, and the constant changes in regulations and technology. It helps development teams make important decisions earlier in the process, reducing costs and time-to-market. Systems engineering can be implemented in phases; it does not require a company to make an “instant switch.” This series of articles will address many aspects of systems engineering, with each entry focusing on a particular aspect or component of the systems engineering approach to device development.
The FDA is also showing more interest in systems engineering for devices. For example, more people at FDA are talking about modeling and simulation as part of a development approach using Model-Based Systems Engineering (MBSE). Successful simulation may help eliminate some clinical trials, resulting in significant reductions in cost and time to bring a device to market. The MBSE topic shall be covered in future articles in this series.
Why Systems Engineering is Important to Product Development
Systems Engineering is not a replacement for product development but rather an enhancement to traditional product development. Using a Systems Engineering approach with traditional product development creates an expanded definition of competitiveness of products by exhaustively examining features, cost, and time-to-market requirements, with a focus on planning early. During early planning, the systems engineering approach focuses on benchmarking existing products to create a clear vision for the product development process and a strong product definition. The end goal in systems engineering is the same as the end goal of traditional product development - to define and deploy a product that satisfies its intended use. When a product development team chooses to implement a systems engineering approach they will avoid making invalid assumptions or omissions, manage ever-changing issues more efficiently, and produce a robust solution to user needs.
In our next article, we will discuss some of the early steps in system engineering including the identification of needs (i.e. User Needs, Regulatory Needs, Business Needs).




