Strategies for Fostering Innovation in Life Sciences
Time to market is only part of the equation when it comes to being competitive in life science industries. The other major factor is differentiation: what does your product do that is inventive and improves outcomes for users and patients beyond the capacities of similar, legally marketed products? To build that value into your product, innovation is key.
However, many organizations struggle with building this in their development teams, and it can be an issue that negatively impacts you in the long run. To foster innovation at your life science organization, you can deploy a handful of strategies.
Communicate your design principles/vision
New ideas and solutions don’t often come out of nowhere; they require a framework within which to operate. With any given design “challenge,” communicating the principles and vision behind it is crucial for idea generation. Coming up with solutions that address the problems you’re trying to resolve or get to the end goal of development is easier when these are established early on.
Encourage collaboration
 It’s very easy, in life science product development, for different working groups to become isolated from each other; your risk teams focus solely on their risk management work while your quality assurance folks are looking only at testing and quality work, and so on. This can be detrimental to your activities in a multitude of ways. In the context of innovation, it can be a significant hindrance to progress.
It’s very easy, in life science product development, for different working groups to become isolated from each other; your risk teams focus solely on their risk management work while your quality assurance folks are looking only at testing and quality work, and so on. This can be detrimental to your activities in a multitude of ways. In the context of innovation, it can be a significant hindrance to progress.
Offering teams opportunities to intermingle and share knowledge, struggles, ideas, etc. encourages greater levels of collaboration. Both in early-stage development and as the project matures, this has major benefits. Potential issues can be identified, insights can be shared, teams can collaborate on entire components and subsystems of your product, and so on.
Allow time for innovation
 If you direct your teams to focus exclusively on product design and risk activities, little to no room is available for them to explore creative ideas and solutions. This is a difficult balance to strike in life sciences, especially since time to market is so crucial. How do you give enough time for innovation without it negatively impacting your timelines?
If you direct your teams to focus exclusively on product design and risk activities, little to no room is available for them to explore creative ideas and solutions. This is a difficult balance to strike in life sciences, especially since time to market is so crucial. How do you give enough time for innovation without it negatively impacting your timelines?
There’s no single answer to this problem, but it often comes down to understanding areas where your team is strongest and where improvement is needed. Where things need to be improved, time and direct effort should be applied to bring competency up; likewise, more freedom and leniency can be granted for areas where your team performs strongest. This offers opportunities for continuous improvement, as well as space to think.
Build failure into your innovation processes
 “Failure” can be a scary word to most organizations, but it doesn’t have to be. Even in your compliance activities, trial and error are necessary parts of the equation; in order to refine your product design and ensure it is safe and effective, consistent risk evaluation, impact analysis, testing, and verification and validation testing have to be performed. Results of these activities can illuminate issues and expose possible design failures which have to be controlled.
“Failure” can be a scary word to most organizations, but it doesn’t have to be. Even in your compliance activities, trial and error are necessary parts of the equation; in order to refine your product design and ensure it is safe and effective, consistent risk evaluation, impact analysis, testing, and verification and validation testing have to be performed. Results of these activities can illuminate issues and expose possible design failures which have to be controlled.
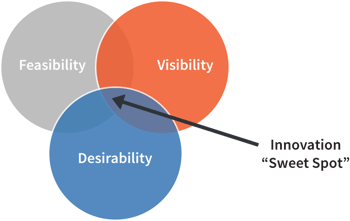
Building similar structures into your innovation processes assists your teams in identifying the feasibility, viability, and desirability of a given idea or solution. Likewise, giving space to ideas that don’t pan out can be very beneficial to your innovation processes. Letting an idea fail and then trying to understand why brings you to important observations that can be incorporated into future activities and development work. Likewise, allowing failure fosters an organizational culture where trial and error are the norm. This can encourage more team members to take that risk and see what happens.
Leverage your compliance activities
Iteration is important during your early-stage activities. By going through successive steps to scope out, understand, test, and refine ideas and solutions, more effective innovations can evolve into your product’s design. However, advancements don’t end as soon as you switch to your design controls and risk management activities—rather, they need to be fostered throughout development.
 Many life science organizations get stuck in this mindset that building their products is a series of sequential steps, and once they reach the next step they don’t look back. While having a basic design sequence set up offers structure to compliance activities, you can make it more fluid and flexible. The majority of creative brainstorming and idea generation occurs during early-stage development, yet compliance and innovation activities should feed into each other throughout the progression of your life science product.
Many life science organizations get stuck in this mindset that building their products is a series of sequential steps, and once they reach the next step they don’t look back. While having a basic design sequence set up offers structure to compliance activities, you can make it more fluid and flexible. The majority of creative brainstorming and idea generation occurs during early-stage development, yet compliance and innovation activities should feed into each other throughout the progression of your life science product.
To leverage your compliance activities for innovation, cyclical thinking and building understanding are key. For example, results of risk analyses can feed back into your tools and processes for further evaluation and problem solving. From there, those insights can be included in your compliance activities and adequately implemented and controlled within the product design.
When it comes to fostering innovation, patience and organization are vital. Good ideas don’t happen overnight, and there has to be space for them to grow. Your development teams already have lots of potential for creating new ideas and solutions—all you have to do is find ways to tap into them.
About Cognition Corporation
At Cognition, our goal is to provide medical device and pharmaceutical companies with collaborative solutions to the compliance problems they face every day, allowing the customer to focus on their products rather than the system used to create them. We know we are successful when our customers have seamlessly integrated a quality system, making day-to-day compliance effortless and freeing up resources to focus on product safety and efficacy.