Reusability: Data Duplication to Accelerate Medical Device Development
Key takeaway: The ability to reuse product data can dramatically reduce the time taken to design, gain regulatory approvals, and ultimately bring new medical devices to market.
Time equates to money in any business, and in the world of medical device design and manufacturing, that can mean BIG numbers!
Depending on the route taken, following FDA regulations, the average cost to bring a medical device to market through the Premarket Approval pathway is $94 million. The less intensive 510(k) process averages out at $31 million.
Taking shortcuts is not an option, not when rules and regulations are so stringent. But that doesn’t mean that device manufacturers need the same level of time and effort to satisfy regulatory demands when bringing a new product to market when they already have access to some of the product data needed.
It’s not just a simple case of “cut and paste,” though. A robust reusability technology framework is needed.
In the context of product design, reusability takes existing documentation, and data to create new and individual products, as well as products in the same family tree, or those that share similar features and functionalities.
Rather than re-entering existing information to design a new product or a variation of an existing product, it’s also possible to leverage previously created detailed information. Being able to do this can have a direct impact in terms of scaling a product line—or even an entire company.
Let’s look at how this works and at some of the other benefits.
Zero-Margin For Error
Liability for defects, inconsistent quality, fluctuating supplier costs, increased globalization, and device regulations all pose challenges for the medical device industry. The margin of error approaches zero for medical devices being brought to market. But if defects are not discovered and corrected during development, companies could face patient injury or death, plus legal consequences.
That’s why data handling, and the documentation required to meet compliance and verify the production process, is so critical. It has to be done accurately and efficiently.
Regardless of how you approach the development process, any opportunity to save time is huge. But because of the nature of medical devices, considerations such as risk mitigation, the potential to cause harm, and regulatory compliance are paramount concerns; these need to be thoroughly addressed no matter how much time they take. Compliance is crucial to the pathway for clearance and approval by the US Food & Drug Administration (FDA) and Notified Bodies (and Competent Authorities) in the EU, with registration required in the UK through the Medicines and Healthcare products Regulatory Agency (MHRA). And again, the more accurate and clear your documentation is, the faster your device can move through the approval process.
Integrating Risk, Requirement, & Test Management
It’s surprising how many companies rely on legacy tools such as Excel spreadsheets to manage their product development and compliance at every stage. This can be laborious at best, and at worst can result in erroneous data. This is particularly evident when it comes to trace tables, which can be a huge undertaking in a program like Excel.
Providing reports containing errors could derail product development and manufacturing, and manually building traces based on inaccurate data creates errors on top of errors that compromise the regulatory approval process
Device manufacturers need a system that truly unifies risk, requirement, and test management for a structured, automatically traceable, fully auditable, design control process. That’s why Cognition developed Compass. Powered by the Cockpit Platform, it’s the only solution that takes a templated, guided approach to device development and includes built-in, enforced processes for quality management and custom document generation functionality - meeting reporting and regulatory submission needs.
Ready-made Reusability
Compass is an 'off-the-shelf, out-of-the-box system' SaaS solution purpose-built to connect data across all functional areas of medical device product development and to automate a complex workflow between those interconnections.
It provides an adaptable set of templates for the entire product design control process: risk, requirement, and test management, from user needs to validation. It maintains documentation and supports submissions as well as provides automatic generation of the master trace matrices for the Design History File (DHF).
The key to the effectiveness of these solutions is the ability to create once, and in the case of Compass, use ready-made or created libraries and templates that allow parts of the project data to be reused. The advantage of reusability kicks in especially based on product variants, i.e., when a product line has different versions but much of the same functionality. The product team can retrace their steps using the various design stages and essentially duplicate the compliance steps up to a certain point, then adapt the plan only for the new or different parts of the product.
Take Personal Protective Equipment (PPE) as a use case, specifically disposable medical gloves. Although all gloves have the same attributes (5 fingers, entry for the hand, different sizes), the chemical composition may be different depending on how a medical glove is used, or they may even come in different colors. Using Compass, the user needs (such as “color”) can be easily altered at scale, using data from the product libraries’ data on the original product.
Additionally, users can create templates from existing designs, giving them a head start by using existing data. Take, for example, user needs and the design inputs that satisfy them. If the next product is an iteration of the existing product, the user needs and design inputs are probably similar, and the design inputs are not too different either. If you can identify the common data, you can kickstart a project from a library that contains all these items already.
Slight changes to some of the attributes of these items might need to be made, but the overall data structure is already there.
So, where is the big difference between Cognition’s solutions and Excel spreadsheets? Because the medical device industry is strictly governed by regulations, there is a need to extrapolate risk mitigation. If a user wants to implement a change in certain requirements in three different areas in Cockpit Enterprise, this is a simple task represented by a data point. If that data point is changed, it is not changed in just one place; it is automatically changed anywhere that it exists across the project.
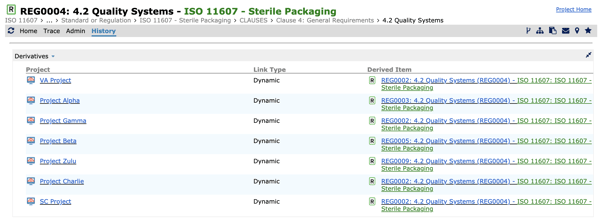 Figure 1: Example of the reuse of a regulation across multiple projects.
Figure 1: Example of the reuse of a regulation across multiple projects.
By contrast in Excel, as the spreadsheets are built on cells, each cell needs the change to be typed in individually and there is no way to ensure it is changed in all places, no single source of truth. Yes, you could just cut and paste Excel data from one spreadsheet to another, but this adds even more complexity and potential for error.
If you multiply a given change across a hundred products, over thousands of requirements, you can easily lose the integrity of your data in Excel and necessitate hundreds of additional hours updating the previous requirements. But using a structured data environment, if you change one item, that change can be propagated wherever it is used.
Built-in, Automated Process and Workflow
Although in Excel you can build lookup tables, you can’t incorporate different process flows that can be enforced and verified. In Compass and Cockpit Enterprise, this process workflow can be built into the templates, meaning that access and editability depend on workflow state, user role, and ownership. No other tools on the market have this functionality.
Automated processes make it easy to apply quality best practices to ensure every change is accounted for and nothing falls through the cracks: a master map and single point of truth where all actions and their impacts across a product’s development lifecycle are recorded.
Faster Time To Market. With Less Risk
Essentially, we’re talking about structured data management. When users need only enter something once, they can then be sure that the data, no matter how many places it exists within a project, is always up-to-date and correct. If a change is made to the data, it changes wherever it exists across the project.
By contrast, with Excel, for example, there is always the danger that a reused bit of text is not exactly as it is elsewhere or has been overlooked when a change was made back in an earlier development stage. Manual processes and the lack of linkage or connection between all compliance data makes product development—and critical traceability—highly error-prone, time-intensive, expensive, and complex.
However, when technology and process meet to create and maintain connections between diverse data, products can get to market faster with less risk. That’s a huge benefit for any medical device company.
Learn how Compass or Cockpit can get you there!





