Is Your Life Science Organization Leveraging These 4 Risk Management Tools?
Whether your life science organization is growing or already well-established, you understand the importance of risk management in product development. For both compliance and product fidelity, you need to be sure as much risk as possible is designed out of your product.
However, it seems like there are millions of risk management tools and activities out there. How do you know which ones will prove the most beneficial? While the answer is mostly dependent on your organization’s capacities and needs, there are four particular risk management tools that you should think about adopting. Not only are they widely used across the industry, but some are also recommended in FDA guidance.
1. Failure Mode Effects Analysis
Failure Mode Effects Analysis (FMEA) is a well-proven risk management tool. FMEA activities help your development teams evaluate possible failure modes of a given process and the resulting impacts on your product and/or users. Particularly suitable for evaluating and controlling process risks, this risk tool breaks down risk analysis for complex systems into manageable steps. As a result, your risk management teams can identify and develop mitigations for elements and operations within your product’s system that could otherwise compromise safety and effectiveness.
Conducting FMEA exercises can be most constructive early in the development process. By looking at early-stage failure modes—known, predicted, or otherwise—the resulting controls can be applied to the product design. While FMEAs should be done throughout the development process and into the postmarket environment, their value in early-stage development is worth keeping in mind.
2. Fault Tree Analysis (FTA)
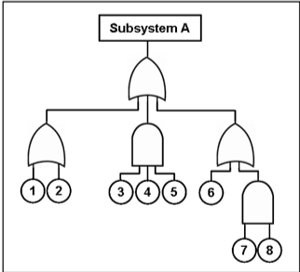 Fault trees offer your risk management teams a visual representation of failure modes. Causal chains with logical operators (AND, OR, etc.) describing each level in the tree show ways toward identifying root causes of failure modes. Using FTA exercises, your risk teams can identify how failure modes occur more in depth.
Fault trees offer your risk management teams a visual representation of failure modes. Causal chains with logical operators (AND, OR, etc.) describing each level in the tree show ways toward identifying root causes of failure modes. Using FTA exercises, your risk teams can identify how failure modes occur more in depth.
FTAs are particularly practical in risk management because they offer a graphical way of understanding faults and failures related to your product’s design. While most risk tools present data in matrices, FTAs show a top-down informational trace. Your risk teams can use the tool for more rapid understanding of event sequences that both lead to failures and influence related hazards. When paired with other risk management tools, FTAs can be a highly effective way of assessing and controlling risk in your product.
3. Use Error Analysis (UEA)
 While FMEAs and FTAs are beneficial for identifying failure modes related to processes, design, etc., use error presents an entirely new dynamic. Because cognition and perception are much harder to predict and control for, identifying the resulting actions and use errors they influence is a difficult task. There’s also a multitude of external factors that need to be taken into account. Anything from time of day and use environment to levels of alertness and physical limitations can change how a user interacts with your product.
While FMEAs and FTAs are beneficial for identifying failure modes related to processes, design, etc., use error presents an entirely new dynamic. Because cognition and perception are much harder to predict and control for, identifying the resulting actions and use errors they influence is a difficult task. There’s also a multitude of external factors that need to be taken into account. Anything from time of day and use environment to levels of alertness and physical limitations can change how a user interacts with your product.
Use Error Analysis (UEA) helps your risk teams scope out these factors and connect them to potential use errors. From the data the UEA generates, you can identify mitigations, develop risk controls, and re-evaluate for any residual risk. The ability to assess a multitude of influencing factors is what makes the UEA particularly powerful for life science risk management; by seeing all the pieces that could contribute to use error, more dynamic risk controls can be developed and implemented. While it’s impossible to control for all usability risks, the UEA brings use error that much more under control.
4. Preliminary Hazard Analysis
 Conducting preliminary hazard analysis (PHA) exercises in your organization’s risk management activities can be a very useful exercise. Whereas other risk tools look at the failure mode first, PHAs look at the hazards, hazardous situations, and harms those failure modes expose users to. This, in conjunction with other risk tools, prioritizes failure modes, use errors, and hazards that expose users and patients to the highest levels of risk.
Conducting preliminary hazard analysis (PHA) exercises in your organization’s risk management activities can be a very useful exercise. Whereas other risk tools look at the failure mode first, PHAs look at the hazards, hazardous situations, and harms those failure modes expose users to. This, in conjunction with other risk tools, prioritizes failure modes, use errors, and hazards that expose users and patients to the highest levels of risk.
When conducting PHA activities, the emphasis is on understanding either known or anticipated hazards. You can generate these hazards through postmarket data, logical reasoning, or otherwise. Like all risk tools, it’s important to run through iterative PHA exercises because knowledge of all possible hazards is inherently limited. However, applying information that’s already available or predictable/foreseeable can help in coming up with pragmatic risk controls early in development.
Risk Tools Should Work Together
While these four risk management tools are helpful on their own, using just one of them may not be adequate for all your risk management needs. Instead, consider using multiple risk tools that can interface well together. For example, conducting FTA exercises on use errors identified through UEA activities can illuminate the root cause of that error and where in the user-product interface risk controls would be most valuable. Such combinations of tools not only make your risk management more dynamic, but help you work toward a safer, more effective product.
About Cognition Corporation
At Cognition, our goal is to provide medical device and pharmaceutical companies with collaborative solutions to the compliance problems they face every day, allowing the customer to focus on their products rather than the system used to create them. We know we are successful when our customers have seamlessly integrated a quality system, making day-to-day compliance effortless and freeing up resources to focus on product safety and efficacy.




